Creating a Healthier Tomorrow for Patients with Kidney and Cardiorenal Diseases
Tenapanor for Hyperphosphatemia
Leveraging our scientific insights and discovery of the primary pathway of phosphate absorption, we have developed tenapanor for the treatment hyperphosphatemia, an electrolyte disorder in which there is an elevated level of phosphate in the blood.
Hyperphosphatemia is a nearly universal condition in more than 550,000 Americans with chronic kidney disease (CKD) on dialysis, and a major risk factor for Cardiorenal morbidity and mortality. Despite the widespread use of phosphate binders, the only currently available class of therapy for this condition, a significant proportion of patients are unable to achieve and maintain target phosphorus levels.
Tenapanor is an investigational first-in-class, phosphate absorption inhibitor with a unique mechanism of action that blocks paracellular absorption of phosphate in the GI tract, creating the potential to completely change the hyperphosphatemia treatment paradigm.
In clinical studies to date (see below), this novel approach to treating hyperphosphatemia has been shown to reduce phosphorus with one small pill taken twice a day, as both a monotherapy and as part of a dual-mechanism approach. On June 30, 2020 we submitted a New Drug Application to the U.S. FDA for the approval of tenapanor for the control of serum phosphorus in adult patients with CKD on dialysis. If approved, we intend to commercialize tenapanor for hyperphosphatemia in the U.S.
Clinical Trials
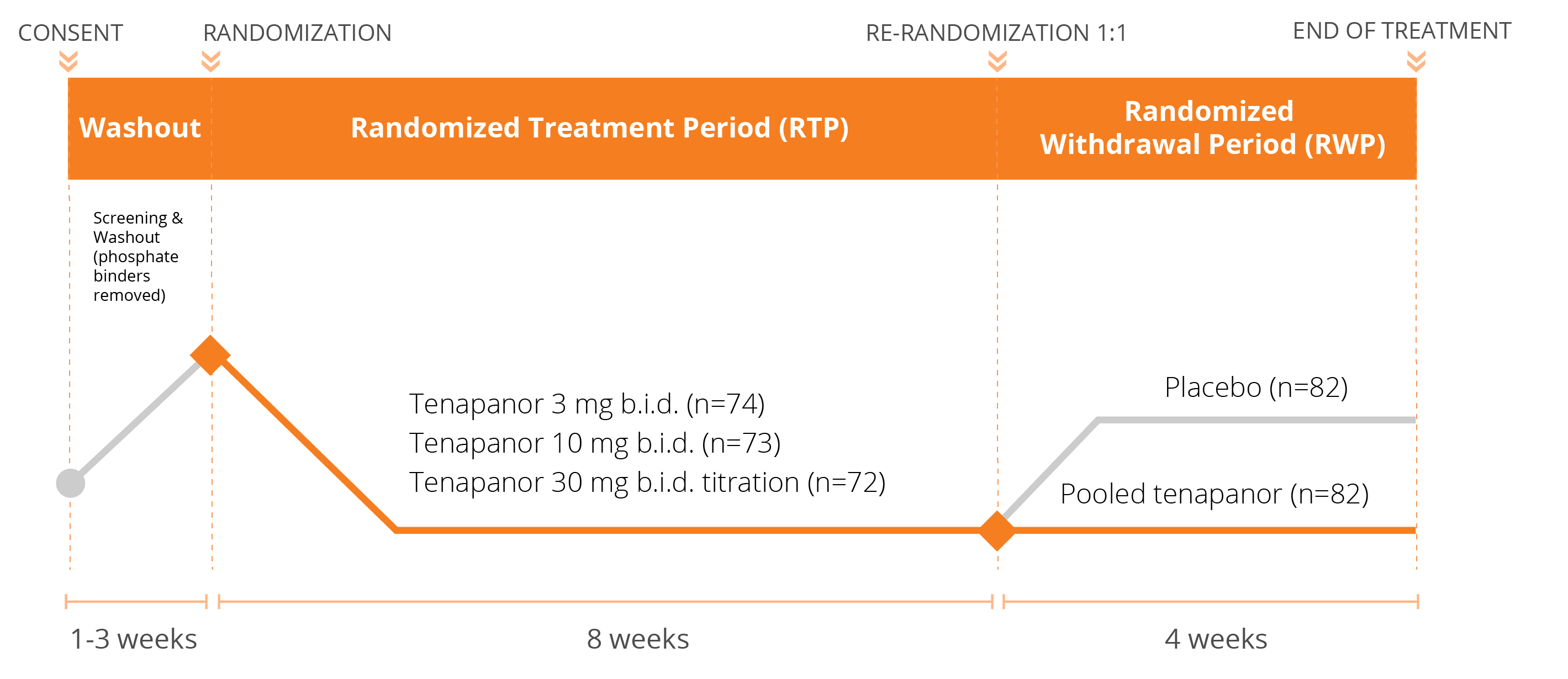
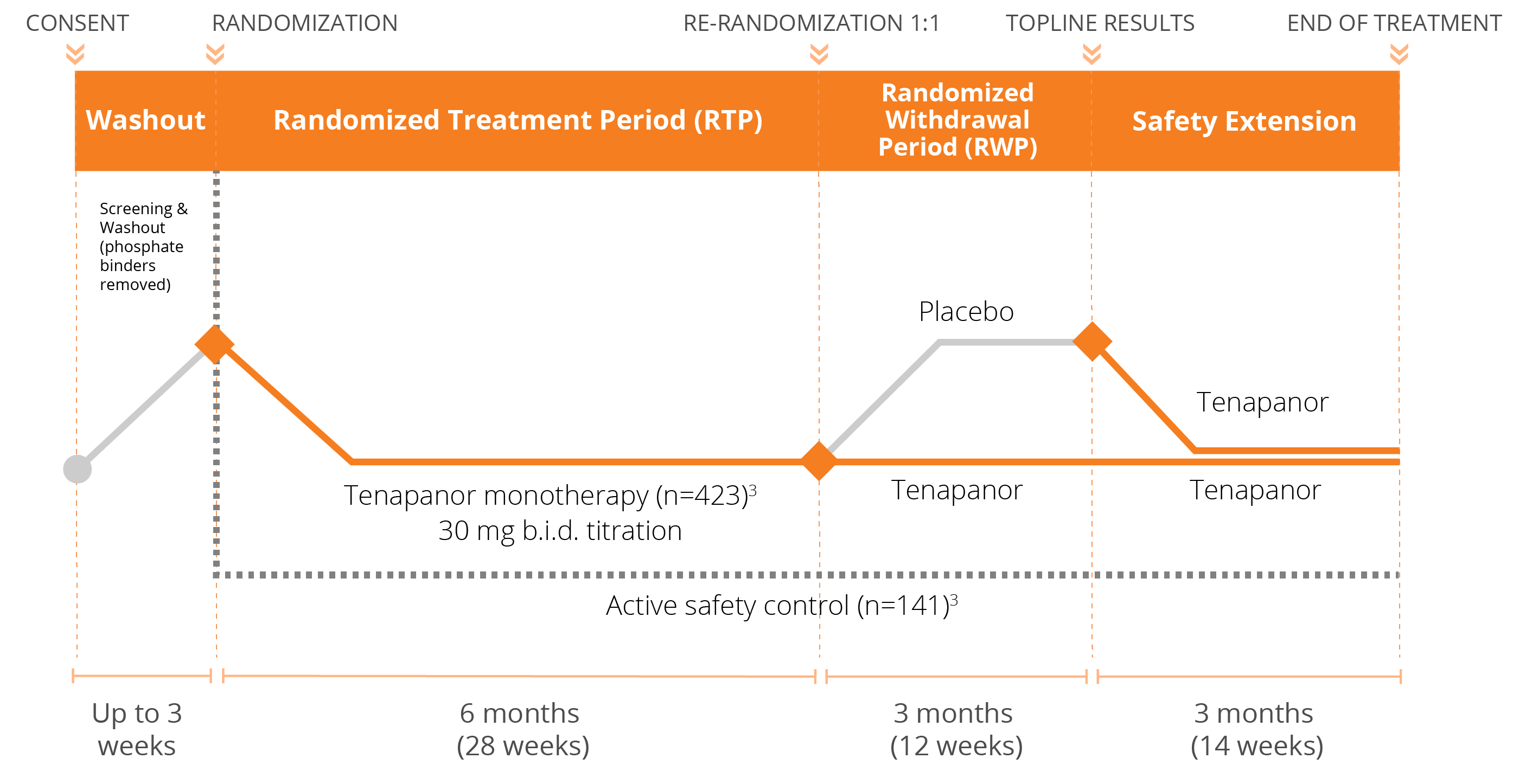
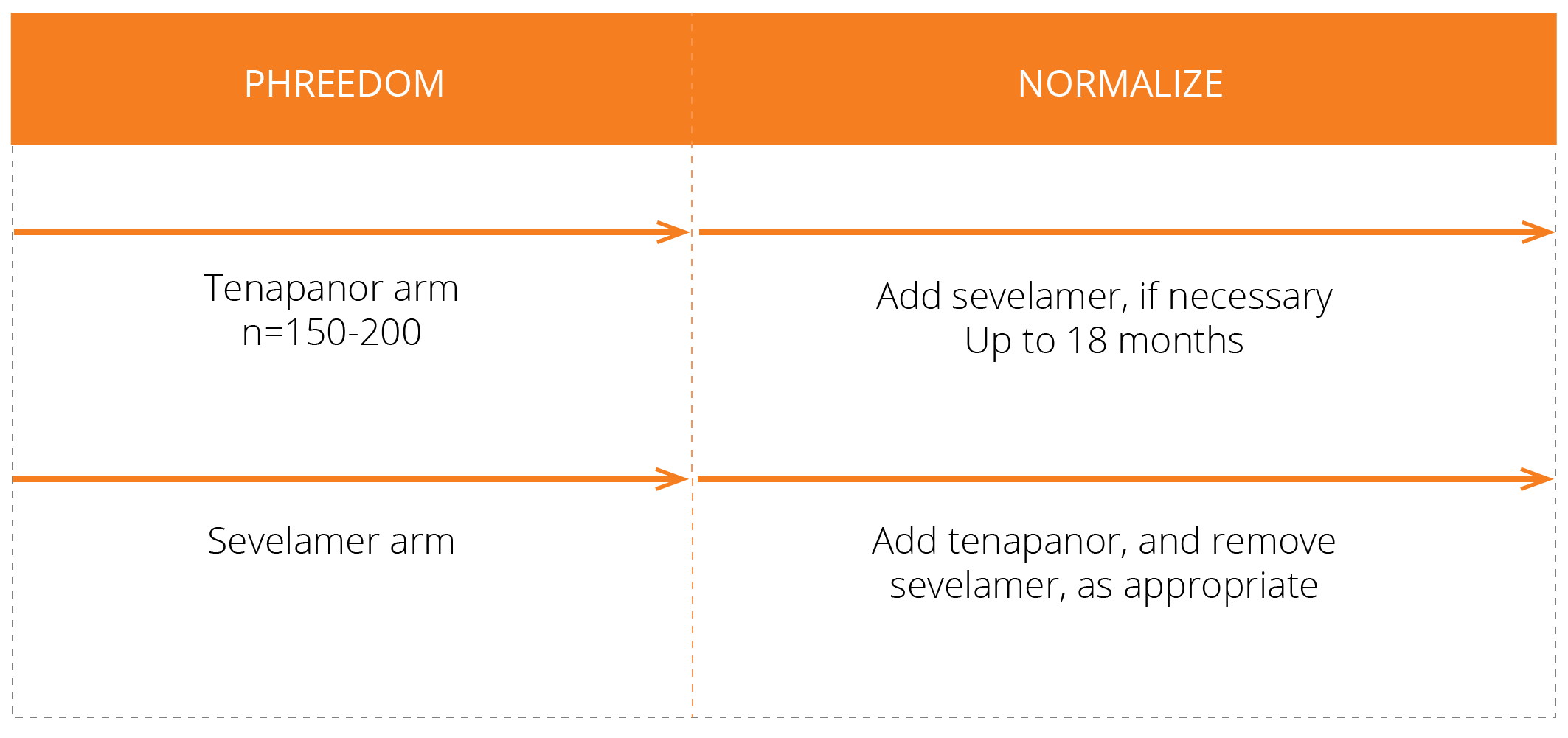
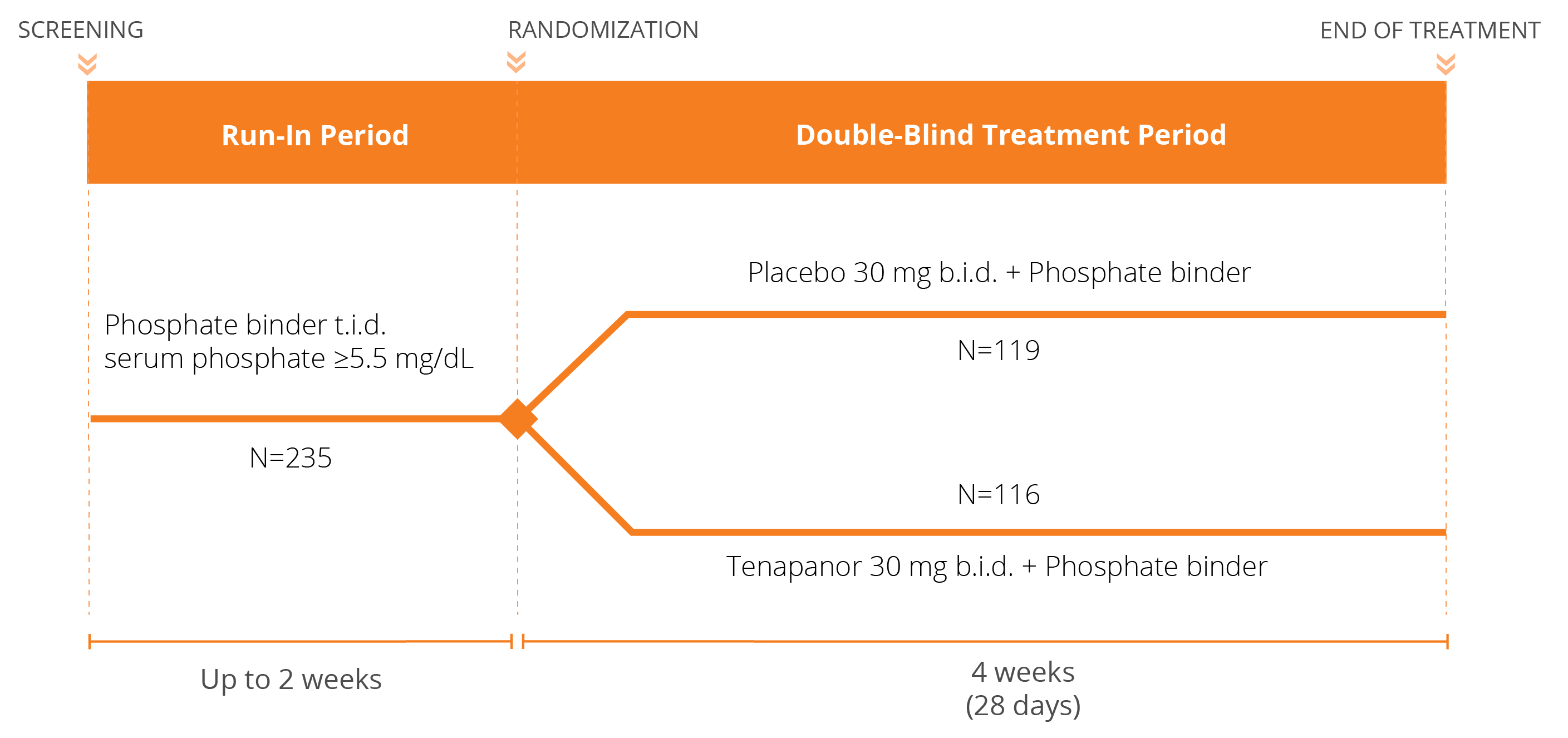
We have conducted three Phase 3 clinical trials evaluating the efficacy and safety of tenapanor for the control of serum phosphorus in adult patients with CKD on dialysis: two monotherapy trials (BLOCK and PHREEDOM) and one dual mechanism trial (AMPLIFY). We are also in the midst of an open label extension study evaluating the ability to achieve normal phosphorus levels (2.5 – 4.5 mg/dL) with tenapanor.
Tenapanor New Drug Application (NDA) submitted on June 30, 2020 for the control of serum phosphorus in adult patients with CKD on dialysis



Our global partners currently include Kyowa Kirin, Fosun Pharma, and Knight.
- In November 2017, we executed a license agreement with Kyowa Kirin for the development and commercialization of tenapanor for cardiorenal diseases and conditions associated with them, including hyperphosphatemia, in Japan.
- In December 2017, we executed a license agreement with Shangai Fosun Pharmaceutical Industrial Development Company Limited for the development and commercialization of tenapanor for Irritable Bowel Syndrome with Constipation (IBS-C) and hyperphosphatemia related to chronic kidney disease in China.
- In March 2018, we executed a license agreement with Knight Therapeutics, Inc. to commercialize tenapanor in Canada for the treatment of IBS-C and hyperphosphatemia. Tenapanor is currently available for the treatment of IBS-C in Canada.
RDX013 for Hyperkalemia
Our research platform has enabled us to identify mechanisms for amplifying potassium secretion, leading to the discovery of our lead candidate in the RDX013 program that targets potassium secretion through the lumen of the gut. Via this mechanism, we believe the candidate has the potential to lower levels of serum potassium to treat hyperkalemia (a condition of high serum potassium levels).
Hyperkalemia is a common problem in patients with kidney and heart disease and can cause a significantly increased risk of death because of the potential for heart conductance issues. There are estimated to be approximately 2 million people in the U.S. with CKD and/or heart failure with hyperkalemia, which remains an underserved market despite the availability of potassium-binding therapies.
Unlike current treatments that bind potassium, our lead candidate in the RDX013 program represents a first-in-class, targeted therapy that could create a completely new treatment paradigm for patients with hyperkalemia if successfully developed.
Other Programs
Based on our successes to date in translating our scientific insights into promising targeted therapies, we are continuing our efforts and applying our expertise to uncover additional breakthroughs to benefit patients suffering from the complications of kidney and Cardiorenal diseases both in-house and in collaboration with our partners.
What is Hyperkalemia?
Hyperkalemia is a potentially life-threatening condition in which blood levels of potassium are elevated above normal. Potassium is a nutrient that is critical to the normal function of nerve and muscle cells, including those in the heart. Normal potassium blood levels are tightly balanced and maintained primarily by the kidneys. For people with chronic kidney disease (CKD), heart failure, and diabetes, and particularly those also taking highly beneficial renin-angiotensin-aldosterone system (RAAS) inhibitors, there is a greater risk of developing hyperkalemia due to side effects and the kidney’s limited ability to keep potassium in balance.
Because of the risk of hyperkalemia, several published guidelines have suggested that physicians should reduce and possibly discontinue RAAS inhibitors in order to manage the risk of hyperkalemia in CKD and heart failure patients. The alternative medications used to control hypertension, including diuretics and calcium channel blockers, are less effective than RAAS inhibitors, particularly in patients with failing kidneys and severe hypertension.
According to the 2015 publication Market Dynamix: Hyperkalemia, released by Spherix Global Insights, U.S. cardiologists reported that of the patients who would benefit from RAAS inhibition, up to 38% of patients with heart failure and up to 55% of patients with both heart failure and CKD are being administered a sub-optimal dose or none at all. Nephrologists reported that at least one-third of patients who would benefit from RAAS inhibition receive a sub-optimal dose or none at all. We believe there is clearly a strong medical need for new medications that control hyperkalemia in order to allow for optimal use of RAAS inhibitors to control hypertension in these patient populations.
It is estimated that 2.1 million people in the U.S. with CKD and/or heart failure have hyperkalemia, which remains an emerging and unaddressed market with today’s treatments.
What is Hyperphosphatemia?
Hyperphosphatemia, a nearly universal condition among patients with CKD on dialysis, is an electrolyte disorder in which there is an elevated level of phosphate in the blood. Hyperphosphatemia is a major independent risk factor for Cardiorenal morbidity and mortality in patients on dialysis. In fact, Cardiorenal disease is the leading cause of death in this patient population and traditional risk factors alone do not explain the high rates of Cardiorenal disease. Hyperphosphatemia has emerged as a predominant and modifiable risk factor for cardiovascular morbidity and mortality, and as such, effective management of serum phosphorus is critical for patients with CKD on dialysis.
While dialysis is the basis for homeostatic electrolyte management, dialysis regimens are unable to successfully remove excess phosphate in order to achieve a neutral phosphate balance. As a result, approximately 80% of patients with CKD on dialysis require phosphate-lowering therapy on top of restrictive, low phosphorus diets.
Despite widespread treatment with currently available therapies, a significant proportion of patients are unable to achieve and maintain target phosphorus levels. While the 2017 KDIGO clinical practice guidelines recommend lowering elevated phosphate levels toward the normal range (2.5-4.5 mg/dL or 0.81-1.45mmol/L), due to the difficulties in managing phosphorus, most clinicians target phosphorus levels between 3.5-5.5 mg/dL (1.13-1.78 mmol/L), based on the 2003 KDOQI clinical practice guidelines. Even the less aggressive targets are often unachievable today with approximately 40% of patients having phosphorus levels >5.5 mg/dL (1.78 mmol/L) in any given month, and approximately 80% of patients unable to consistently maintain phosphorus levels ≤5.5 mg/dL (1.78 mmol/L) over a 6-month period.
Currently available treatments all belong to the class of drugs referred to as phosphate binder therapies. Achieving and maintaining effective phosphate control with phosphate binders is extremely challenging. Phosphate binders act by binding dietary phosphorus in the gut. The binding mechanism requires frequent dosing and often, many large pills in order to bind enough phosphorus, making phosphate binders the largest contributor to excessive pill burden for patients on dialysis. Phosphate binders also tend to be associated with a number of GI side effects including nausea, vomiting, abdominal pain, diarrhea, and constipation.
Here at Ardelyx, we have discovered that the paracellular pathway is the primary mechanism by which dietary phosphorus is absorbed. By developing first-in-class therapeutics to specifically target and block phosphorus absorption through the paracellular pathway, we hope to address what continues to be a significant unmet medical need in patients on dialysis.